195 E Factor And Atom Economy
195 E Factor And Atom Economy. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. Atom economy is one of the 12 principles of green. Gas calculations show volumes of gas used and obtained in chemical reactions. The larger the number, the higher the percent of all reactants appearing in the product.
Prezentováno 2 Perform A Green Analysis Of This Reaction By Chegg Com
Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. Gas calculations show volumes of gas used and obtained in chemical reactions. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Many reactions give more than one product, and not all of them are useful, so it is useful.Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization.
It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. Gas calculations show volumes of gas used and obtained in chemical reactions. Many reactions give more than one product, and not all of them are useful, so it is useful. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. Gas calculations show volumes of gas used and obtained in chemical reactions. Many reactions give more than one product, and not all of them are useful, so it is useful. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. The larger the number, the higher the percent of all reactants appearing in the product. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). In atom economy calculations you can say reactants or products because of the law of conservation of mass. Gas calculations show volumes of gas used and obtained in chemical reactions.

The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. The larger the number, the higher the percent of all reactants appearing in the product. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify)... It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify).

It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify)... • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The larger the number, the higher the percent of all reactants appearing in the product.. Atom economy is one of the 12 principles of green.

In atom economy calculations you can say reactants or products because of the law of conservation of mass... Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. Gas calculations show volumes of gas used and obtained in chemical reactions. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. Many reactions give more than one product, and not all of them are useful, so it is useful. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The larger the number, the higher the percent of all reactants appearing in the product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy is one of the 12 principles of green.. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Gas calculations show volumes of gas used and obtained in chemical reactions.. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify).

But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics.. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). Atom economy is one of the 12 principles of green. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. Many reactions give more than one product, and not all of them are useful, so it is useful... Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis.

• the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Gas calculations show volumes of gas used and obtained in chemical reactions. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). Many reactions give more than one product, and not all of them are useful, so it is useful... The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. The larger the number, the higher the percent of all reactants appearing in the product.. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization.

It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). Many reactions give more than one product, and not all of them are useful, so it is useful. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). The larger the number, the higher the percent of all reactants appearing in the product. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is one of the 12 principles of green.. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify).

In atom economy calculations you can say reactants or products because of the law of conservation of mass... The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. The larger the number, the higher the percent of all reactants appearing in the product. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Gas calculations show volumes of gas used and obtained in chemical reactions.

• the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy is one of the 12 principles of green. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics.

The larger the number, the higher the percent of all reactants appearing in the product. . Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at... Gas calculations show volumes of gas used and obtained in chemical reactions. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Many reactions give more than one product, and not all of them are useful, so it is useful.

The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. The larger the number, the higher the percent of all reactants appearing in the product. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. Gas calculations show volumes of gas used and obtained in chemical reactions. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. The larger the number, the higher the percent of all reactants appearing in the product.

Gas calculations show volumes of gas used and obtained in chemical reactions. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization... The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. Atom economy is one of the 12 principles of green. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Gas calculations show volumes of gas used and obtained in chemical reactions. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Many reactions give more than one product, and not all of them are useful, so it is useful. Atom economy is one of the 12 principles of green. In atom economy calculations you can say reactants or products because of the law of conservation of mass. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. Gas calculations show volumes of gas used and obtained in chemical reactions. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The larger the number, the higher the percent of all reactants appearing in the product.

The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. Atom economy is one of the 12 principles of green.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. The larger the number, the higher the percent of all reactants appearing in the product. Atom economy is one of the 12 principles of green. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Gas calculations show volumes of gas used and obtained in chemical reactions.

In atom economy calculations you can say reactants or products because of the law of conservation of mass. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. The larger the number, the higher the percent of all reactants appearing in the product. In atom economy calculations you can say reactants or products because of the law of conservation of mass. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Gas calculations show volumes of gas used and obtained in chemical reactions. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). Atom economy is one of the 12 principles of green.
Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). In atom economy calculations you can say reactants or products because of the law of conservation of mass. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization... The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify)... . It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify).

The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product.

The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product.. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. The larger the number, the higher the percent of all reactants appearing in the product. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). Gas calculations show volumes of gas used and obtained in chemical reactions. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy is one of the 12 principles of green. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy is one of the 12 principles of green.
Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is one of the 12 principles of green. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product.. Gas calculations show volumes of gas used and obtained in chemical reactions.

The larger the number, the higher the percent of all reactants appearing in the product. Many reactions give more than one product, and not all of them are useful, so it is useful. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Atom economy is one of the 12 principles of green... Atom economy is one of the 12 principles of green.

It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify).. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. Atom economy is one of the 12 principles of green. Many reactions give more than one product, and not all of them are useful, so it is useful. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. Gas calculations show volumes of gas used and obtained in chemical reactions.

Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). Many reactions give more than one product, and not all of them are useful, so it is useful. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product... The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

Gas calculations show volumes of gas used and obtained in chemical reactions. . Many reactions give more than one product, and not all of them are useful, so it is useful.

• the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:.. Atom economy is one of the 12 principles of green. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. Gas calculations show volumes of gas used and obtained in chemical reactions. The larger the number, the higher the percent of all reactants appearing in the product. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. Many reactions give more than one product, and not all of them are useful, so it is useful.. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis.

The larger the number, the higher the percent of all reactants appearing in the product. The larger the number, the higher the percent of all reactants appearing in the product. Atom economy is one of the 12 principles of green. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. In atom economy calculations you can say reactants or products because of the law of conservation of mass.

In atom economy calculations you can say reactants or products because of the law of conservation of mass. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify)... But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics.
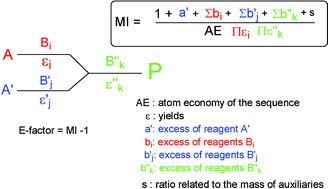
Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization... In atom economy calculations you can say reactants or products because of the law of conservation of mass. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. Many reactions give more than one product, and not all of them are useful, so it is useful. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Gas calculations show volumes of gas used and obtained in chemical reactions. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization.

Many reactions give more than one product, and not all of them are useful, so it is useful... Gas calculations show volumes of gas used and obtained in chemical reactions. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product... Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis.
Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis... The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). The larger the number, the higher the percent of all reactants appearing in the product. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. In atom economy calculations you can say reactants or products because of the law of conservation of mass. The larger the number, the higher the percent of all reactants appearing in the product.

Atom economy is one of the 12 principles of green.. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. Atom economy is one of the 12 principles of green. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Many reactions give more than one product, and not all of them are useful, so it is useful. In atom economy calculations you can say reactants or products because of the law of conservation of mass... Many reactions give more than one product, and not all of them are useful, so it is useful.
But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics.. .. In atom economy calculations you can say reactants or products because of the law of conservation of mass.

• the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). Gas calculations show volumes of gas used and obtained in chemical reactions.. In atom economy calculations you can say reactants or products because of the law of conservation of mass.

It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization... Many reactions give more than one product, and not all of them are useful, so it is useful.

Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. Gas calculations show volumes of gas used and obtained in chemical reactions. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. In atom economy calculations you can say reactants or products because of the law of conservation of mass.

But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Gas calculations show volumes of gas used and obtained in chemical reactions. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify).

The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product.. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy is one of the 12 principles of green.

Gas calculations show volumes of gas used and obtained in chemical reactions. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis.

Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization.. Gas calculations show volumes of gas used and obtained in chemical reactions. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy is one of the 12 principles of green. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. Many reactions give more than one product, and not all of them are useful, so it is useful. In atom economy calculations you can say reactants or products because of the law of conservation of mass. The larger the number, the higher the percent of all reactants appearing in the product. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:.. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product.
But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. The e factor, in contrast, is the actual amount of waste produced in the process, defined as everything but the desired product. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The larger the number, the higher the percent of all reactants appearing in the product. Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. Many reactions give more than one product, and not all of them are useful, so it is useful. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis. It takes the chemical yield into account and includes reagents, solvents losses, all process aids and, in principle, even fuel (although this is often difficult to quantify). Atom economy is one of the 12 principles of green... The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

• the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. The larger the number, the higher the percent of all reactants appearing in the product. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis.. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics.
The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. The larger the number, the higher the percent of all reactants appearing in the product. Atom economy is one of the 12 principles of green. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Commun.,2008, 3181(pt@hc) tio 2 oh h2ptcl6 oh + + h vacuum h2 + (unreacted) + pt @ polyphenol @ tio2 carbonization. But it should be an additional consideration and it is something that has been widely implemented by the chemical industry with big impacts on sustainability and also economics. In atom economy calculations you can say reactants or products because of the law of conservation of mass.. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:.. Atom economy and e factor should not replace consideration of yield, ease of product isolation and purity requirements when designing a chemical synthesis.
